39 structure function claims on dietary supplement labels
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... Constructing structure and function claims - Natural Products INSIDER Structure/function claims describe a nutrient's or dietary ingredient's effect on or maintenance of the structure or function of the body—for example, "calcium builds strong bones" or "antioxidants maintain cell integrity." A disease claim is an express or implied claim to diagnose, mitigate, treat, cure or prevent a disease—for ...
Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism(s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims.
:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3650624/quakerlabel-shelf.0.jpg)
Structure function claims on dietary supplement labels
Demystifying Dietary Supplement Claims part I: What is and What isn't a ... Since the enactment of the Dietary Supplement Health and Education Act (DSHEA) of 1994, FDA has lacked authority to approve structure/function claims (and related general well-being and nutrient-deficiency disease claims) before the dietary supplement products enter the market.However, a manufacturer must submit a notification with the text of the claim to FDA no later than 30 days after ... Should function claims be allowed on dietary supplements? Which claims can or Cannot be made on dietary supplement labels? Basically, dietary supplements cannot make 'disease' claims (for example: 'this supplement shrinks tumors'). Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds ... ''Structure/Function'' Claims in Dietary Supplement Labeling - ResearchGate Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function' claims on...
Structure function claims on dietary supplement labels. Structure/Function Claims | FDA 07.03.2022 · Dietary Supplements. Structure/function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education ... Label Claims for Food and Dietary Supplement - LBS RCS.COM it contains criteria to determine when a labeling statement made about a dietary supplement constitutes a structure/function claim for which no prior fda review is required and when it constitutes a disease-related claim that requires either authorization of a health claim or review under the drug provisions of federal food, drug, and cosmetic … How to Choose High Quality Vitamins and Supplements - Healthline Jul 15, 2020 · Certain circumstances, such as nutrient deficiencies, conditions causing malabsorption, inadequate access to food, and life stages like pregnancy, may make it necessary to add a supplement to your ... Federal Preemption of 'Structure/Function' Claims on Dietary Supplements Congress amended the Federal Food, Drug, and Cosmetic Act (FDCA) with the Nutrition Labeling and Education Act (NLEA) in 1990 and, in 1994, with the Dietary Supplement Health and Education Act (DSHEA), which provided the Food and Drug Administration (FDA) with regulatory authority over dietary supplements and specifically established "standards with respect to dietary supplements ...
Dietary Supplements: An Advertising Guide for Industry Application of FTC Law to Dietary Supplement Advertising A. Identifying Claims and Interpreting Ad Meaning 1. Identifying Express and Implied Claims 2. When to Disclose Qualifying Information 3. Clear and Prominent Disclosure B. Substantiating Claims 1. Overview 2. Ads that Refer to a Specific Level of Support 3. The Amount and Type of Evidence 4. Labeling Guidance: Making Structure/Function Claims the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in... FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, … Background Information: Dietary Supplements - Consumer The label of a dietary supplement or food product may contain one of three types of claims: a health claim, nutrient content claim, or structure/function claim. Health claims describe a relationship between a food, food component, or dietary supplement ingredient, and reducing risk of a disease or health-related condition.
Dietary Supplements - Claims and Labeling Compliance for FDA/FTC Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds strong bones'). A structure/function claim describes the product's role in maintaining the 'structure or function of the body,' or 'general well-being.' Labeling rules are ... Dietary Supplements – Nutrition: Science and Everyday … Dietary supplements can’t be marketed with claims that they can diagnose, treat, cure, mitigate, or prevent any disease; such claims would require the product to be approved by the FDA as a pharmaceutical. Instead, dietary supplements are marketed with health claims or structure/function claims, similar to claims on food labels. Recall from ... structure function claims — FDA Reader — FDA Reader A Structure/Function Claim describe the role of a nutrient or ingredient on the structure or function of the human body. These may appear on the labels of foods, dietary supplements or drugs. Examples of a Structure/Function Claim: "Calcium builds strong bones" "Fiber maintains bowel regularity" Nutraceutical - Wikipedia Depending on its ingredients and the claims with which it is ... may claim to prevent chronic diseases, improve health, delay the aging process, increase life expectancy, or support the structure or function of the body. Dietary supplements. Dietary supplements, such as the vitamin B supplement shown above, are typically sold in pill form. In the United States, the …
Small Entity Compliance Guide on Structure/Function Claims (2022) An example of an acceptable claim is "a good diet promotes good health and prevents the onset of disease" or "better dietary and exercise patterns can contribute to disease prevention and better health.". An example of a disease claim is "Promotes good health and prevents the onset of disease" because the claim infers that the product itself will achieve the intended effect.
Structure/function claims in dietary supplement labeling: not all of ... Structure/function claims in dietary supplement labeling: not all of these claims need to be submitted to FDA and accompanied in labeling by the DSHEA disclaimer. McNamara SH(1). Author information: (1)Hyman, Phelps & McNamara, P.C., Washington, D.C, USA PMID: 11758558 [PubMed - indexed for MEDLINE] MeSH Terms
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels...
Dietary Supplements: Structure/Function Claims Fail To Meet Federal ... A manufacturer must notify FDA when it uses structure/function claims, and a product label must include a disclaimer stating that FDA has not reviewed the claim and that the product is not intended to diagnose, treat, cure, or prevent any disease. HOW WE DID THIS STUDY. We analyzed structure/function claims for a purposive sample of 127 dietary ...
Chapter 2 Practice Test Flashcards | Quizlet Which of the following statements regarding claims on food or supplement labels is NOT true? A. The word "healthy" may not be used on products that are high in fat or sodium. B. Structure/function claims must always use "may" or "might" when describing the potential benefit of the product. C. A food must provide at least 10% of the DV per ...
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in...
FDA Issues Final Rules for Structure/Function Claims for Dietary ... (4.) Blumenthal M. FDA Proposes New Rules on Dietary Supplement Structure-Function Claims: Agency Redefines "Disease" in What Critics Call an Attempt to Limit Claims and Weaken DSHEA. HerbalGram. 1998. 43:26-28.57. (5.) Commission on Dietary Supplement Labels. Report to the President, the Congress, and the Secretary of Health and Human Services.
Dietary Supplements | Consumer Advice 01.11.2011 · Dietary supplements also can carry claims about the effect of a substance on maintaining the body's normal structure or function — "Product B promotes healthy joints and bones" — but must include the disclaimer: "This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent …
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
An examination of structure-function claims in dietary supplement ... Dietary supplement advertising cannot claim a causal link between the product and the treatment, prevention, or cure of a disease unless manufacturers seek approval from the FDA for a health claim. Manufacturers can make structure-function (S-F) claims without FDA approval linking a supplement to a …
Dietary Supplements for Weight Loss - Health Professional Fact … Approximately 15% of U.S. adults have used a weight-loss dietary supplement at some point in their lives; more women report use (21% ... where it supports their structure and function. Calcium is required for vascular contraction and vasodilation, muscle function, nerve transmission, intracellular signaling, and hormonal secretion . The Recommended Dietary …
Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
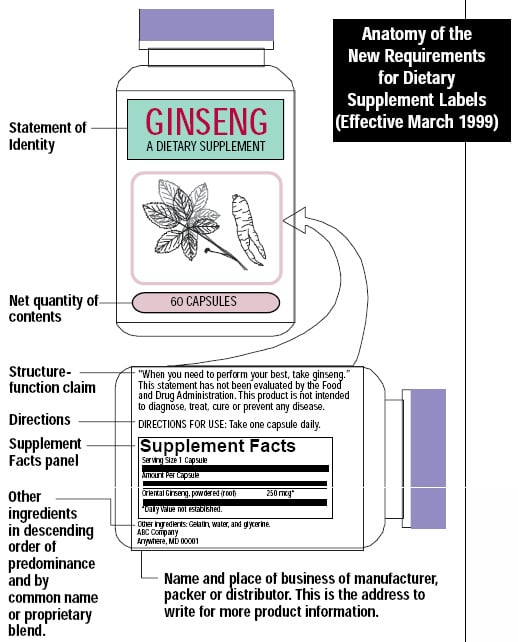
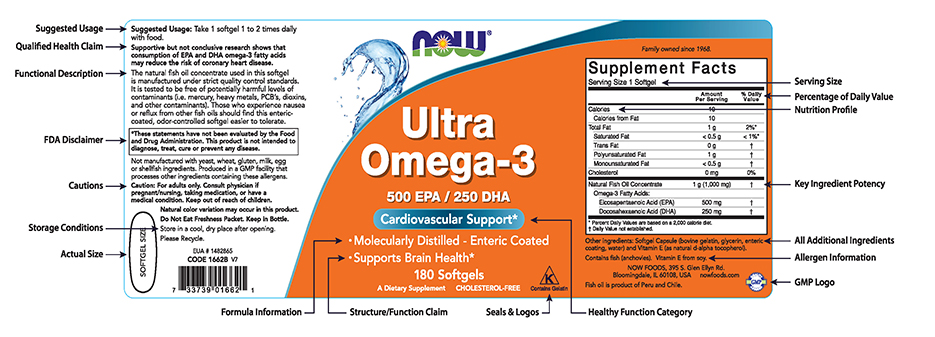
PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the
Structure/Function Claims in Dietary Supplement Labeling: Not All of ... One important means of promoting dietary supplements is the inclusion, in label ing, of claims about the impact on the structure or function of the human body. These claims are known as "structure/function" claims. The author has observed the evolu tion of the Food and Drug Administration's (FDA's) regulation of dietary supplement
OIG Report Finds that Structure/Function Claims on Dietary Supplement ... The OIG's study reviewed 378 structure/function claims on the labels of 127 dietary supplements to determine whether the claims complied with current FDA regulations. The OIG found that 66 of the 104 manufacturers voluntarily submitted documentation substantiating the claims on their products' labels.
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims
Solved Question 21 Structure/function claims on a dietary - Chegg question 21 structure/function claims on a dietary supplement o must acknowledge that the fda supports the claims that are misleading can lead to the product being taken off the market prove that the supplement can prevent a disease prove that the supplement can treat a disease o have to be approved, as done for drugs, before they can appear on …
''Structure/Function'' Claims in Dietary Supplement Labeling - ResearchGate Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function' claims on...
Should function claims be allowed on dietary supplements? Which claims can or Cannot be made on dietary supplement labels? Basically, dietary supplements cannot make 'disease' claims (for example: 'this supplement shrinks tumors'). Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds ...
Demystifying Dietary Supplement Claims part I: What is and What isn't a ... Since the enactment of the Dietary Supplement Health and Education Act (DSHEA) of 1994, FDA has lacked authority to approve structure/function claims (and related general well-being and nutrient-deficiency disease claims) before the dietary supplement products enter the market.However, a manufacturer must submit a notification with the text of the claim to FDA no later than 30 days after ...



























Post a Comment for "39 structure function claims on dietary supplement labels"